Abstract
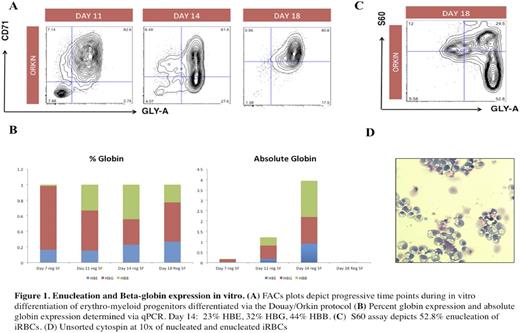
Human induced pluripotent stem cells (hiPSCs) hold tremendous promise for disease modeling and the development of novel therapeutic treatments for sickle cell anemia (SCA). Such models are practical systems for drug screening and to examine the effects of gene editing. hiPSCs can theoretically produce all cell types including erythroid cells. However, in vitro modeling of SCA with hiPSCs has been hampered by their inability to differentiate into terminally-mature, enucleated, beta globin-expressing red blood cells. Here, we describe strategies to improve in vitro and in vivo models of SCA using these hiPSCs. We generated hiPSCs from sickle cell patients with hemoglobin SS disease seen at our hematology clinic at Boston Children's Hospital. Using a cocktail of transcription factors that promote self-renewal and multipotency expressed under the control of a doxycycline-regulated promoter (ERG, HOXA9, RORA, SOX4, MYB), we generated conditionally immortalized hematopoietic progenitors that serve as a renewable source of robust erythroid cells in vitro. Erythroid progenitors differentiated from these lines underwent globin-switching once transplanted into immunodeficient mice with a 27% induction of beta globin expression . Concurrently, we further improved in vitro differentiation protocols to generate 30-40% beta-globin-expressing, erythroid cells with an enucleation rate of 20-50%. In future studies, we hope to employ hiPSCs to test the therapeutic hypothesis that genetic manipulation of BCL11A, a master regulator of fetal hemoglobin (HbF) expression, will ameliorate sickling. The generation of hiPSC-derived SCA models will be critical in broadening the current understanding of the molecular mechanisms of this disease, the development of improved pharmacological treatments and autologous cell therapy for the treatment of SCA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal